泮托拉唑钠干混悬剂处方前研究毕业论文
2020-07-11 18:17:39
摘 要
泮托拉唑是在临床运用的第三代质子泵抑制剂,可以使质子泵丧失活性及泌酸功能,用于消化性溃疡等各种胃肠道疾病的医治。本课题研究了泮托拉唑钠肠溶干混悬剂的制备,该剂型不仅保留了原来肠溶片剂的治疗效果,而且可以根据患者需求分剂量,其散度大,在胃肠道中容易吸收且刺激小,生物利用度高,除此以外还极大的扩大了适用人群,不仅适合于常规成人,而且适合各年龄段的儿童使用,特别适合吞咽有困难的老人和小孩使用。
本课题对泮托拉唑钠肠溶干混悬剂进行了以下研究:
- 处方前研究:调查泮托拉唑钠原料药的稳定性,最终显示泮托拉唑钠对湿度、温度敏感,原料药本身不是很稳定,在设计处方时需要避免高温、高湿的环境。考查了泮托拉唑钠在不同介质中稳定性的差异,结果表明泮托拉唑钠在pH1.2的溶液中即时分解,pH6.8中缓慢分解,在水中分解较少,流动相中较稳定,所以药物在酸性条件下较不稳定,溶液测定时尽量在1h内完成,避免样品分解。药物与辅料彼此作用显示,主药与各辅料之间不会相互影响,原辅料相容性良好,适合制剂研究。
- 体外分析方法建立:建立了高效液相色谱法测定泮托拉唑钠的体外释放度的方法,最终表明,空白辅料不会对测定形成影响;精密度良好;回收率试验结果良好;释放度测定方法结果准确,可靠性高。建立了高效液相色谱法测定泮托拉唑钠的含量的方法,结果表明,空白辅料不会对测定造成干扰;精密度良好;回收率试验结果良好;泮托拉唑钠8小时内在溶剂[0.001mol/L氢氧化钠溶液-乙腈(1:1)]中稳定性良好;该含量测定手法结果准确,可靠性强。
关键词:泮托拉唑钠 混悬剂 高效液相法 质子泵抑制剂
ABSTRACT
Pantoprazole is the third generation proton pump inhibitor used in clinic, which can inactivate proton pump and secrete acid, and can be used to treat gastrointestinal diseases such as peptic ulcer. In this study, the preparation of pantoprazole sodium for delayed-release oral suspension was studied. The dosage form not only retained the therapeutic effect of the original enteric-coated tablet, but also could be divided into different doses according to the needs of the patients. It is easy to absorb in the gastrointestinal tract and it hassmall irritation to the gastrointestinal tract with high bioavailability. Pantoprazole sodium made into oral suspension, in addition to the above advantages, but also has easy to swallow, suitable for children, the elderly and patients with difficulty taking swallow the advantages.
In this study, pantoprazole sodium enteric dry suspension was studied as follows:
Pre-prescription study:Investigating the stability of pantoprazole sodium bulk drug, and finally showing that pantoprazole sodium is sensitive to humidity and heat, and the bulk drug itself is not very stable, so it is necessary to avoid high temperature and humidity when designing prescriptions.
( 1 ) the differences of stability of pantoprazole sodium in different media were investigated. the results showed that pantoprazole sodium was decomposed immediately in solution with ph 1.2, slowly in ph 6.8, less in water and more stable in mobile phase, so the drug was unstable in acidic conditions, and the solution was determined within 1h as far as possible to avoid sample decomposition. The interaction between drug and auxiliary materials showed that the main drug and auxiliary materials would not affect each other, and the compatibility of auxiliary materials was good, so it was suitable for preparation research.
Establishment of in-vitro analysis method: a method for determining the in-vitro release of pantoprazole sodium by high performance liquid chromatography was established, and finally showed that blank excipients would not affect the determination. Good precision; The recovery rate test results are good. The method for determining the release degree has accurate results and high reliability. A method for the determination of pantoprazole sodium by high performance liquid chromatography was established. the results showed that blank excipients would not interfere with the determination. Good precision; The recovery rate test results are good. Pantoprazole sodium has good stability in solvent [ 0.001 mol / l sodium hydroxide solution - acetonitrile ( 1: 1 ) ] for 8 hours. This method is accurate and reliable.
KEYWORDS:Pantoprazole sodium Suspension High performance liquid chromatography Proton pump inhibitors
目录
摘要 I
ABSTRACT II
第一章 文献综述 1
1.1泮托拉唑钠研究概述 1
1.1.1泮托拉唑钠的理化性质 2
1.1.2泮托拉唑钠的作用机制 2
1.1.3泮托拉唑钠的药代动力学 2
1.2泮托拉唑钠的临床应用 3
1.2.1治疗消化性溃疡 3
1.2.2治疗上消化道出血 3
1.2.3治疗反流性食管炎 4
1.2.4泮托拉唑在其它方面的研究 4
1.3泮托拉唑钠的研究现状及发展前景 4
1.3.1泮托拉唑钠国内外上市情况 4
1.3.2泮托拉唑钠市场销售情况 5
1.3.3泮托拉唑钠肠溶干混悬剂的发展前景 6
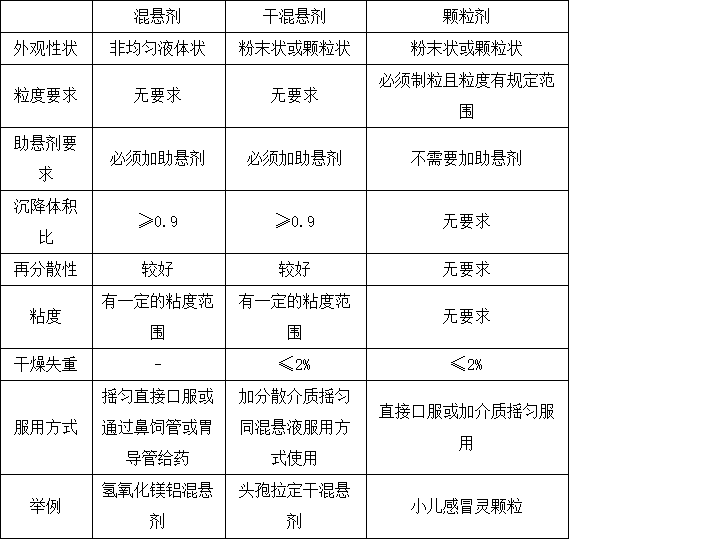
1.4 干混悬剂的概述 6
第二章 泮托拉唑钠肠溶干混悬剂处方前研究 8
2.1试验部分 9
2.1.1试验材料 9
2.1.2试验仪器 9
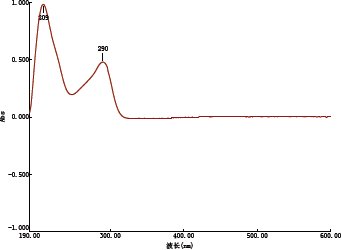
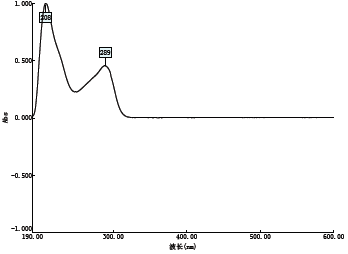
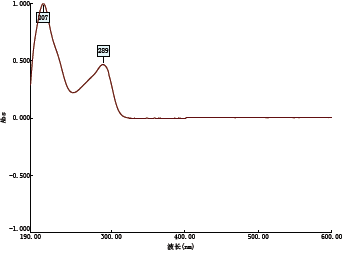
2.1.3 泮托拉唑钠在不同溶剂中的紫外吸收 9
2.1.4 泮托拉唑钠在不同介质中的溶解度 11
2.1.5 泮托拉唑钠在不同介质中的稳定性 12
2.2 泮托拉唑钠原料药的稳定性 13
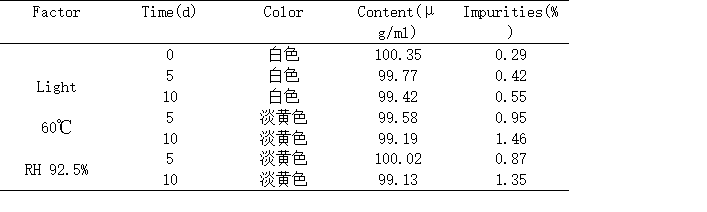
2.2.1各种因素对药物稳定性的影响 13
2.2.2含量变化考察 13
2.3 有关物质检查 14
2.3.1色谱条件与方法 14
2.3.2破坏试验 15
2.4 原辅料相容性试验 16
2.5 本章小结 19
第三章 体外分析方法的建立 19
3.1试验部分 19
3.1.1试验材料 19
3.1.2试验仪器 20
3.2释放度测定方法的建立 20
3.2.1色谱条件 20
3.2.2空白辅料干扰实验 20
3.2.3线性与范围 22
3.2.4进样精密度考察 23
3.2.5回收率试验 23
3.2.6释放度测定条件的选择 24
3.3 含量测定方法的建立 24
3.3.1色谱条件 24
3.3.2空白辅料干扰试验 25
3.3.3线性与范围 26
3.3.4精密度考察 27
3.3.5回收率试验 28
3.3.6溶液稳定性试验 29
3.4 本章小结 29
3.4.1泮托拉唑钠释放度测定方法的建立 29
3.4.2泮托拉唑钠含量测定方法的建立 29
参考文献 30
第一章 文献综述
1.1 泮托拉唑钠研究概述
随着社会的发展,时代的进步,人们的生活习惯也发生了许多变化,许多人为了应酬沾染了烟酒,部分上班族为了投入到忙碌的工作而忽略了饮食的健康,许多青少年热衷于晚睡晚起,不吃早餐,这些行为都给胃肠道带来了很大的负担。如今,胃肠道疾病越来越普遍。质子泵抑制剂(PPI, proton pump inhibitor)在该类疾病的治疗上发挥了重要的作用,越来越受医患的青睐,如奥美拉唑、泮托拉唑、雷贝拉唑、艾普拉唑、兰索拉唑等[1]。
质子泵抑制剂可以抑制质子泵的泌酸功能,质子泵与其在酸性环境中的产物相结合,导致质子泵的活性丧失,抑制了胃酸的分泌[2];H2受体拮抗剂也是临床上用来抑制胃酸分泌的,与其相比,质子泵抑制剂能够迅速起效并长时间发挥抑酸作用,特别是在夜间也能一直发挥药效,与此同时,还能阻断乙酰胆碱、胃泌素等导致的胃酸分泌反应[1]。
相关图片展示:

您可能感兴趣的文章
- 用于重复性光热/热力学协同治疗的NIR-II 光响应抗菌凝胶外文翻译资料
- 氧化石墨烯/银/胶原涂层的光动力和物理作用协 同杀死细菌外文翻译资料
- x,β-不饱和羰基化合物的区域选择性自由 基x-硼酰化直接合成0-硼酰羰基分子外文翻译资料
- 通过光氧催化作用实现有机硼合成的新型自由基硼化途径外文翻译资料
- 光导的单电子转移过程在烯烃与氮杂环卡宾硼烷的硼基化反应中作为- -种授权的基本原理外文翻译资料
- 用于数字光处理3D打印的可重复打印聚合物外文翻译资料
- 1, 6-烯基自由基硼化/环化级联反应合成硼处理的杂环和碳环外文翻译资料
- 羟基环戊烯酮的Morita-Bayllis-Hillman反应研究外文翻译资料
- 莫米洛替尼的新型实用合成路线外文翻译资料
- 用从突变的PrPGApx04中分离出的青霉素G酰化酶高效合成β-内酰胺类抗生素外文翻译资料




