二氧化硅磁性微球的制备毕业论文
2020-04-26 12:47:26
摘 要
目前,在化学领域中,制备性能突出、 能应用于多个领域的核-壳结构的粒子引起了广大研究者的兴趣 [1~3] 。在外加磁场的作用下,二氧化硅磁性微球能快速分离,具有超顺磁性的磁学性质;还具有良好的生物相容性,可以作为生物活性物质的载体;高的化学稳定性;大的比表面积等多种特点。近年发展了一些制备二氧化硅磁性复合材料的新方法如溶胶凝胶法、气溶胶高温分解法、超声合成法、模板法、反相微乳液法等[4]。本篇文章主要以溶胶凝胶法来描述。
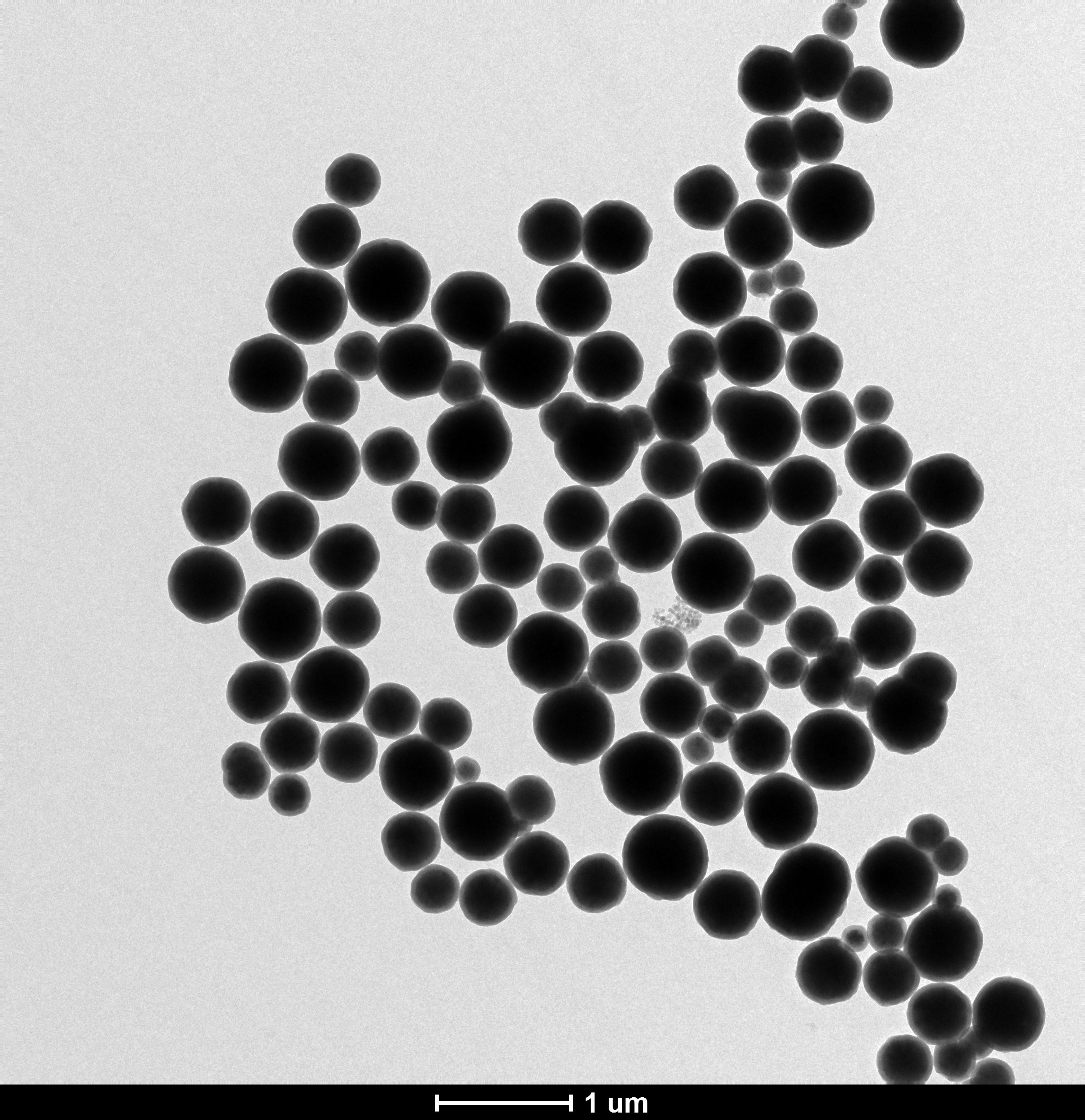
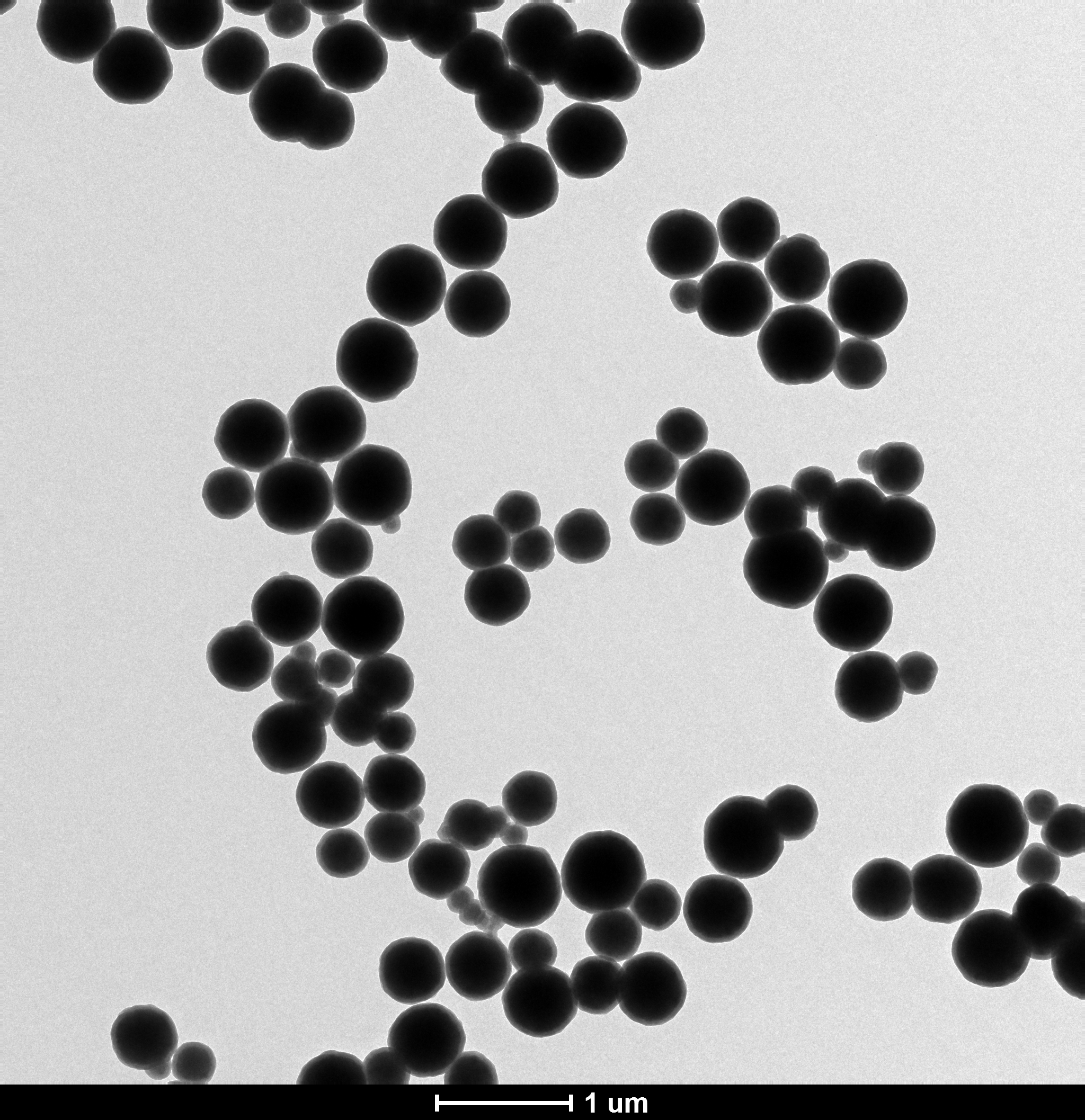
以FeCl3·6H2O、聚乙醇、聚乙二醇、醋酸钠等为主要原料,生成Fe3O4磁性颗粒。然后在Fe3O4磁性颗粒中加入氨水、正硅酸乙酯等,水解、缩合形成Fe3O4/SiO2磁性微球。最后再通过透射电子显微镜和扫描电子显微镜对产物进行表征。实验结果表明:Fe3O4/SiO2磁性微球的粒径随加入反应体系中氨水和TEOS用量的增加而变大;随乙醇和水体积比的增加粒径先变大,而后变小,再又变大。
关键词:Fe3O4 SiO2 磁性材料 溶胶凝胶法
Preparation of Silica Magnetic Microspheres
Abstract
At present, in the field of chemistry, the preparation of particles with outstanding performance and can be applied to many fields of core-shell structure has attracted the interest of researchers [1~3]. The silica magnetic microspheres not only have the magnetic properties of superparamagnetic at room temperature and can be quickly separated under an external magnetic field; but also have good biocompatibility and can be used as carriers for biologically active substances; high chemical stability; high specific surface area and other characteristics.In recent years, some new methods for preparing silica magnetic composite materials have been developed,such as sol-gel method, reverse microemulsion method, aerosol pyrolysis method, ultrasonic synthesis method, template method, etc.[4]. This article is mainly described by the sol-gel method.
Fe3O4 magnetic particles are formed by using FeCl3·6H2O, polyethanol, polyethylene glycol, sodium acetate and as main raw materials. Then, ammonia water, ethyl orthosilicate and something else are added into the Fe3O4 magnetic particles to hydrolyze and condense to form Fe3O4/SiO2 magnetic microspheres. Finally, the product was characterized by transmission electron microscopy and scanning electron microscopy. The experimental results show that the particle size of Fe3O4/SiO2 magnetic microspheres increases with the increase of ammonia and TEOS in the reaction system. With the increase of ethanol and water volume ratio, the particle size becomes larger first, then becomes smaller and then becomes larger.
Keywords: Fe3O4 SiO2 magnetic material Sol-gel method
目录
摘要...............................................................I
ABSTRACT........................................................II
第一章 绪言.........................................................1
1.1 磁性纳米材料的介绍.........................................1
1.1.1 磁性纳米材料的研究过程...............................1
1.1.2 纳米材料的四个效应...................................2
1.1.3 纳米材料可用的表征方法...............................3
1.2 Fe3O4/SiO2磁性微球的分子构成及在医学上的运用................3
1.2.1 Fe3O4/SiO2磁性微球的分子构成..........................3
1.2.2 Fe3O4/SiO2磁性微球在医学上的运用......................4
1.3 实验课题的提出.............................................5
第二章 Fe3O4/SiO2磁性微球的制成....................................6
2.1 引言............. ....... ....... ....... ...................6
2.2 实验部分............................. .....................6
2.2.1 实验药品及仪器............ ... ... ...................6
2.2.2 实验步骤.............................................7
2.2.3 样品的表征.......... ..... ..........................8
第三章 实验的结果与总结............................................9
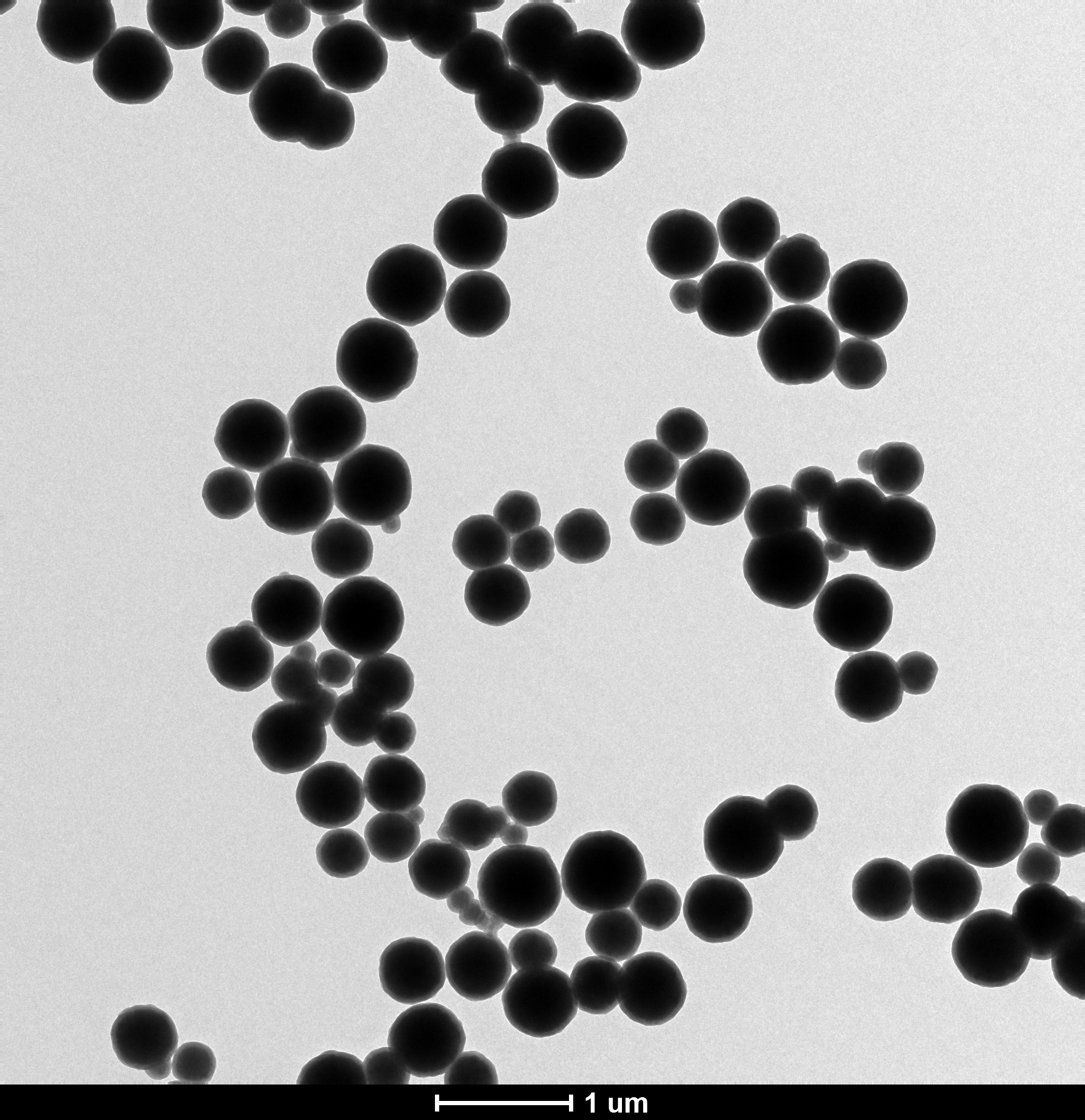
3.1 Fe3O4纳米颗粒的SEM分析...................................9
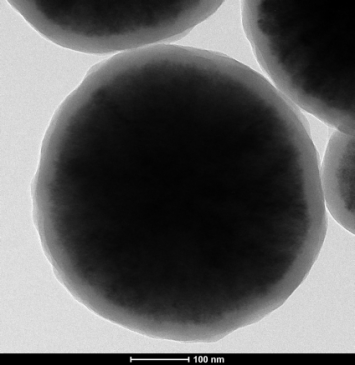
3.2 Fe3O4/SiO2磁性微球的TEM分析...............................9
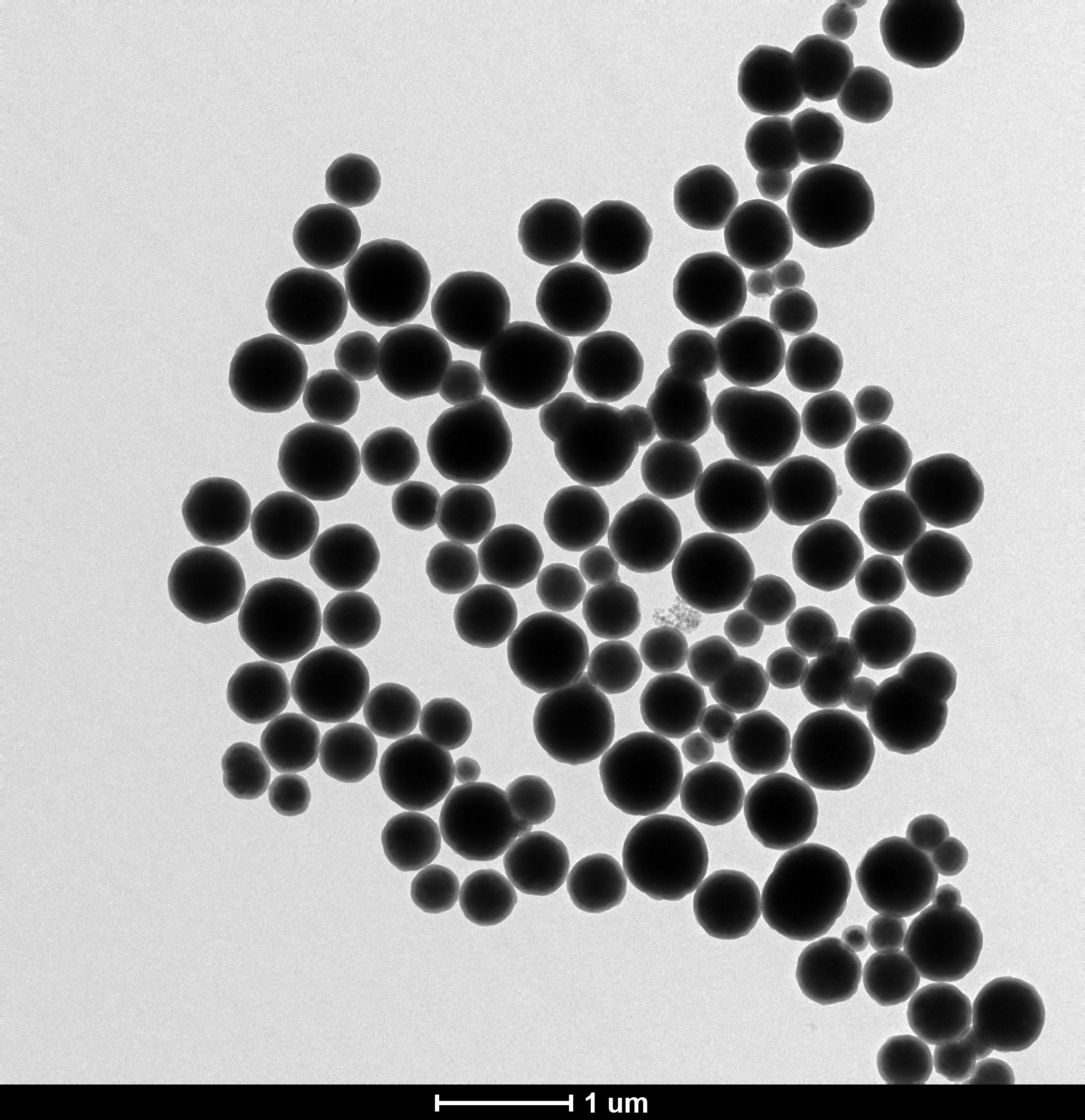
3.3 Fe3O4/SiO2磁性微球的SEM分析..............................10
3.4 Fe3O4和Fe3O4/SiO2磁性微球的FTIR分析.......................10
3.5 反应条件对Fe3O4/SiO2磁性微球的影响........................11
第四章 本文总结...................................................14
参考文献...........................................................15
致 谢..............................................................17
第一章 绪言
相关图片展示: